CPM Seminar
First-Principles Approach for Investigations of
Structural and Electronic Properties and Energy Level Alignment at Aqueous
Semiconductor Interfaces
James Muckerman
Chemistry Department
Brookhaven National Laboratory
Water splitting using semiconductor-based heterogeneous photocatalysis plays
a key role in a promising path to clean and sustainable energy production.
Domen's group has reported that the band gap of GaN can be reduced to absorb
visible instead of UV light by alloying GaN with ZnO. The band-gap-narrowed
GaN/ZnO alloy is an efficient visible-light photocatalyst, although microscopic
models for reaction sites and mechanisms remain as important open questions.
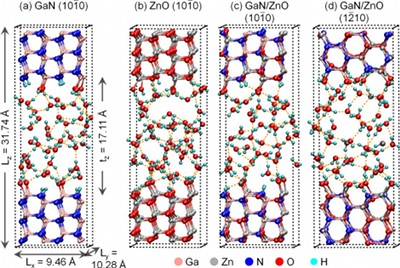
Using density-functional-theory-based molecular dynamics, we investigate
the microscopic structural and electronic properties of aqueous interfaces
of nonpolar Wurtzite facets of GaN, ZnO, and representative GaN/ZnO alloy
structures. We find that water adsorption is substantially dissociative. At
the equilibrated interfaces, most of the surface anions are protonated,
while many surface cations are bonded to hydroxide ions. Surface N-sites
show stronger basic character and are protonated more readily than surface
O-sites. All surface Ga atoms are bonded to hydroxide ions while about 50%
of surface Zn atoms are bonded to hydroxide ions. Our earlier work suggests
that water oxidation at the GaN-water interface is driven by the localization
of photogenerated holes on the adsorbed hydroxides. Additionally, the
hard-wall interface presented by the semiconductor imparts ripples in the
density of the water. Beyond a 3 Å distance from the semiconductor surface,
the water exhibits a bulk-like hydrogen bond network and oxygen-oxygen radial
distribution function. Taken together, these characteristics represent the
resting (or “dark”) state of the catalytic interface.
 Another important issue is that the relative alignment of the semiconductor
band edge and the corresponding redox level in the solvent for a target
reaction determines thermodynamically whether photoexcited carriers in the
semiconductor can drive the reaction and with what range of overpotential.
This influences the design of electrochemical devices for solar energy
harvesting. In particular, it is an unavoidable constraint in the search for
materials that can serve both as efficient absorbers of the solar spectrum
and to supply electrons and holes with sufficient energy to drive relevant
reactions, e.g., the hydrogen evolution reaction or the water oxidation
reaction. I will present the results of our recent studies of these issues,
as well as results from complementary models of the water oxidation reaction
at a GaN photoanode.
Another important issue is that the relative alignment of the semiconductor
band edge and the corresponding redox level in the solvent for a target
reaction determines thermodynamically whether photoexcited carriers in the
semiconductor can drive the reaction and with what range of overpotential.
This influences the design of electrochemical devices for solar energy
harvesting. In particular, it is an unavoidable constraint in the search for
materials that can serve both as efficient absorbers of the solar spectrum
and to supply electrons and holes with sufficient energy to drive relevant
reactions, e.g., the hydrogen evolution reaction or the water oxidation
reaction. I will present the results of our recent studies of these issues,
as well as results from complementary models of the water oxidation reaction
at a GaN photoanode.
Thursday, December 11th 2014, 15:30
Ernest Rutherford Physics Building, R.E. Bell Conference Room (room 103)
|